We are living in strange times. That much is certain.
What isn’t certain, however, is what the world will look like on the other side of this pandemic. For example, leading health experts are suggesting that handshakes will forever be a thing of the past. No doubt, other social norms will be set aside for public health reasons as well.
In the meantime, everyone needs to adapt in real-time to the realities of the current pandemic. Many people are working from home, family and friend meet-ups are being done virtually, and homemade bread is being baked like never before.
Not only are individuals changing their daily routine to combat COVID-19, but entire industries are pivoting as well.
MDR (Smartly) Delayed
Recently, the European Commission proposed the delay of the new Medical Device Regulations (MDR), which was set for May of 2020. The reason for the proposal is simple enough; medical device companies should be focused on getting their much-needed products into hospitals and clinics where healthcare professionals desperately need them.
While the changes that MDR provides is still important—as a lot of the new regulations deal with protecting consumers—delaying these new guidelines is necessary to keep the flow of essential medical devices through the supply chain and into hospitals.
On April 17th, 2020, this proposal was voted on by the European Parliament and passed by a total of 693 ‘yes’ votes to 1 ‘no,’ with two absentees.
“Given the current pressure on national health authorities and manufacturers of medical devices, there is a fear that there could be shortages or delays in getting the medical devices needed to fight COVID-19, were they to follow the new rules of the Medical Device Regulations from May this year,” the Parliament said.
When Will the EU MDR be Implemented?
In order to give companies that are working hard to get their products to market during this pandemic enough time to make the necessary changes for MDR compliance, MDR is being postponed a full year. It was originally scheduled to take effect on May 26th, 2020, but will instead take effect on May 26th, 2021.
How to Enable Your Company to be Ready for MDR Compliance By May 2021
This delay in the roll-out of the new MDR guidelines allows companies to lock down their new processes to ensure they adhere to MDR compliance before the new May 2021 date.
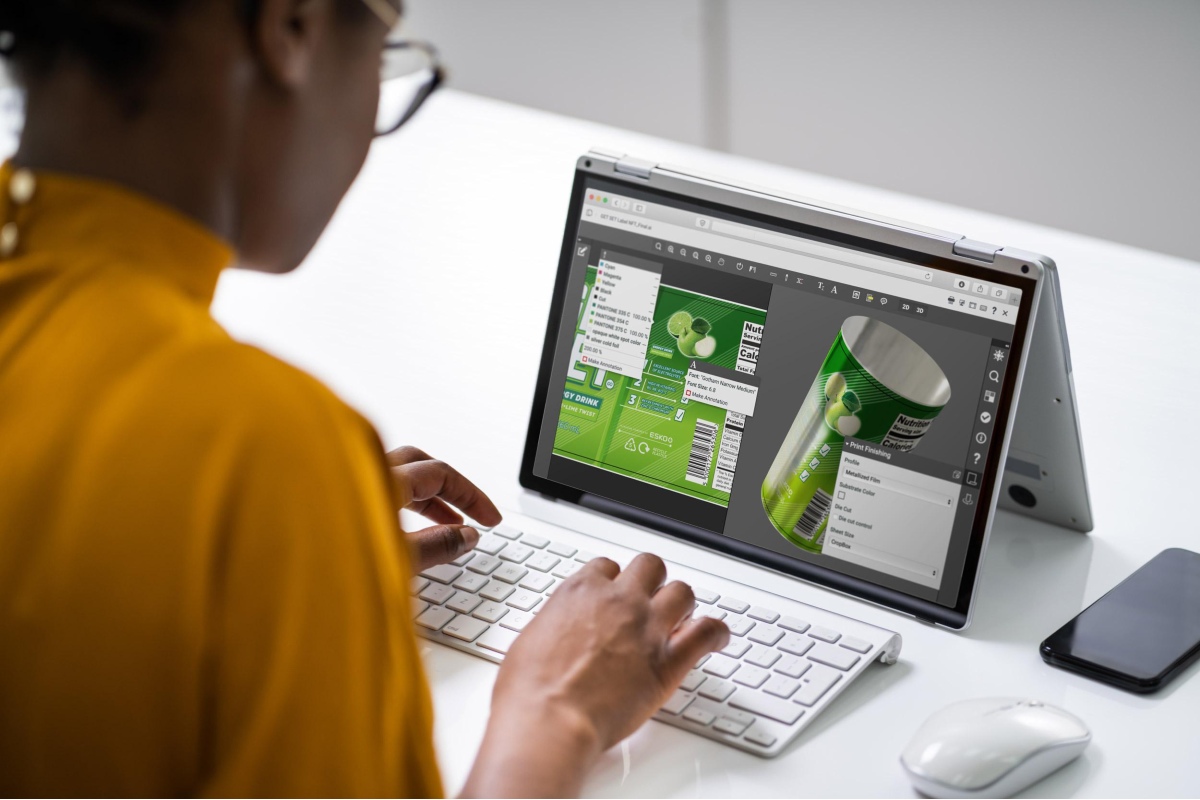
Perhaps the best way to achieve compliance is by incorporating state-of-the-art packaging workflow software into your processes. By upgrading your software, you can streamline your packaging design and creation, making it much easier to address any necessary label changes as a result of the new regulations.
Packaging workflow software can also help automate changes that need to be made to artwork to save time and reduce the chances for copy and paste errors.
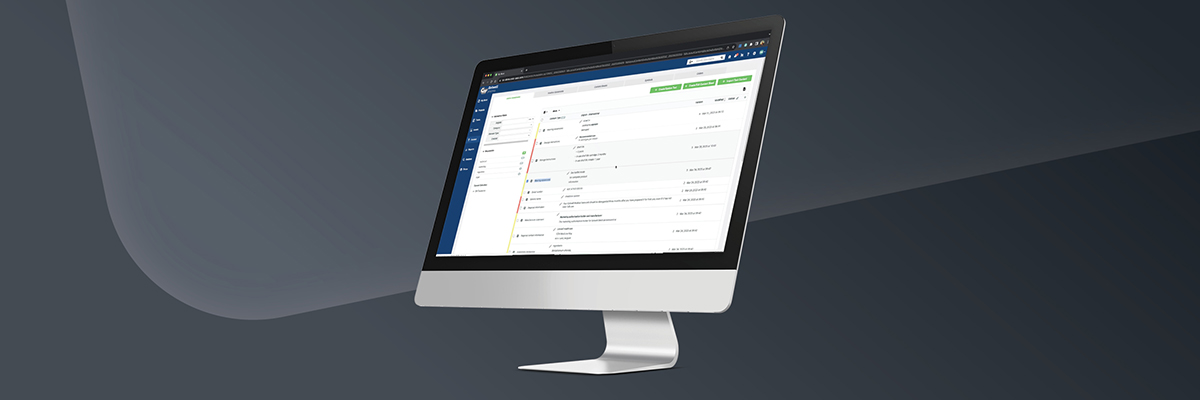
WebCenter is one of the top label and artwork workflow management solutions available today. With this software, you’ll be able to swiftly handle any label changes that come your way due to changing regulations.
WebCenter’s capabilities include:
- Project and task specification
- Dynamic forms and collaboration tools
- File management and asset library, which includes: technical keylines, artwork, graphics, logos, images
- Packaging Content Management, which includes: copy, nutritional fact tables, ingredients, etc.
- Easily invite unregistered users to the project through an approval link
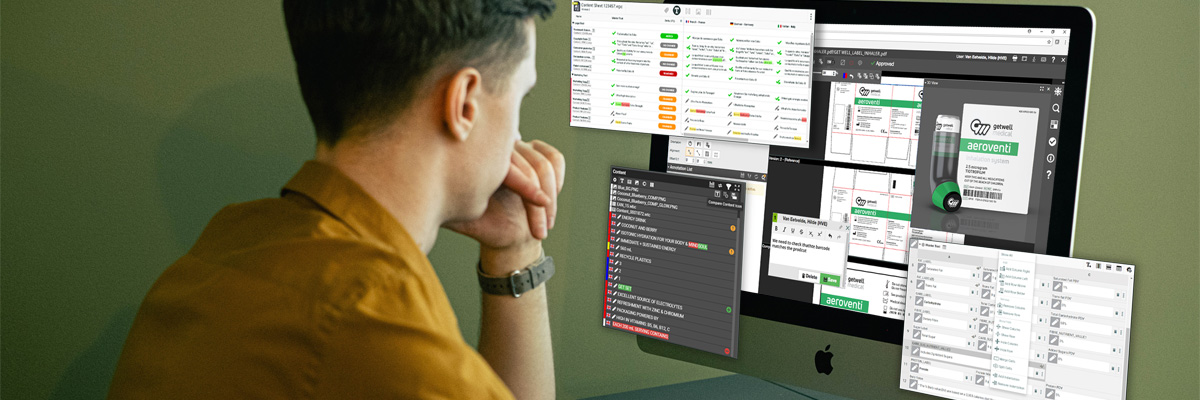
- Version control and history tracking
- Easy-to-use viewing and annotation tools
- View, mark-up, and approve CAD files, PDF files, and image files with WebCenter’s high-resolution viewer
- 3D viewer
- Automated artwork update capabilities
For all your other packaging design needs, Esko has you covered. Check out our full offering for brands looking to level up their packaging game here!